The term "VCI" stands for Volatile Corrosion Inhibitor.
Corrosion inhibitor compounds vaporize from the paper or film. They are attracted to the charged surface of the metal by virtue of their polar orientation.
///
The VCI molecules align on the surface of the metal to a depth of 3 to 5 molecules. This layer of molecules passivates the charged surface and creates a barrier that prevents oxidation.
The corrosion cell (the flow of electrons in the metal and the flow of ions in the electrolytic surface layer) is unable to establish itself.
Corrosion is halted.
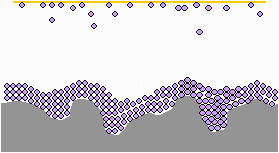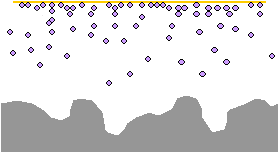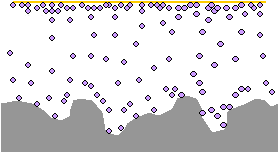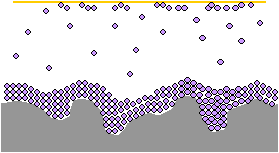
The VCI molecules migrate into recesses and hard to reach areas on even the most complex shapes.
The molecules build up on the metal surface until a continuous barrier has formed on the metal part.
When metals are wrapped or packaged in VCI, the chemicals volatilize in the packaging environment, forming a protective molecular layer on the surface of the metal.
This protective layer serves as a barrier, preventing moisture, salt, dirt, oxygen, and other corrosion causing materials from depositing directly on the metal and causing rust and corrosion.




